Supercapacitors Explained
Supercapacitors (SCs) play a crucial role as electrochemical energy storage devices that enable the reversible adsorption and desorption of ions at the interfaces between electrode materials and electrolytes. The unique properties of supercapacitors allow for fast charge-discharge rates, longer life cycles, high power, and high energy density, making them a superior alternative to conventional capacitors and batteries. It is important to emphasize that supercapacitors offer the best of both worlds, combining the instant charging capabilities of capacitors with the ability to store large amounts of energy like batteries. This is made possible by the much bigger effective surface area of their electrodes and the significantly smaller distance between them as also the chemistry of their electrolytes. As a result, supercapacitors are capable of storing and releasing energy quickly, making them ideal for high-power delivery or energy storage applications. Additionally, supercapacitors offer the advantage of being charged and discharged any number of times without any significant degradation in their performance. Moreover, their minimal internal resistance means that they store and release energy with close to 100 percent efficiency, making them an attractive option for energy storage applications
IMAGE of SC and explanation of how it works:
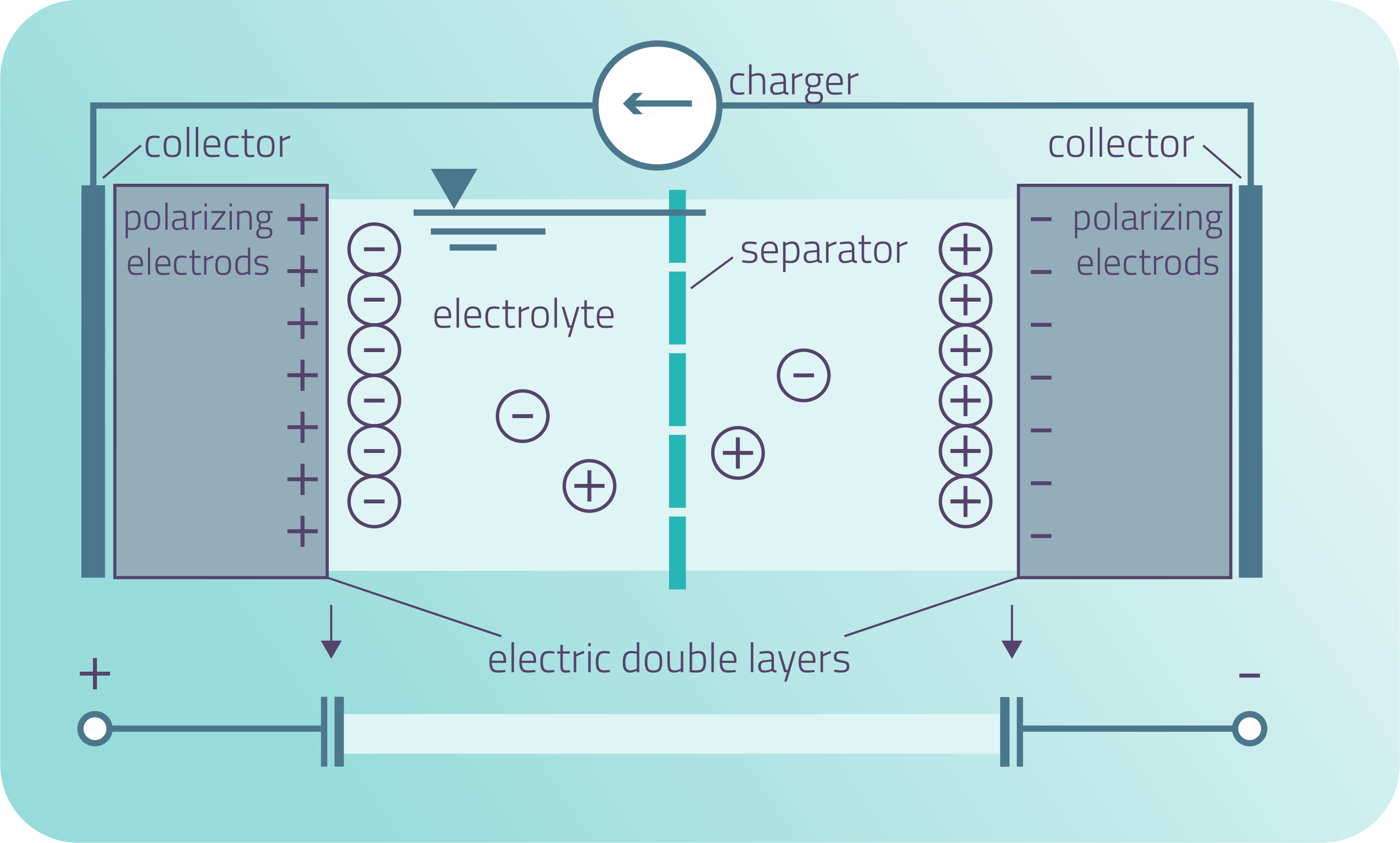
The supercapacitor comprises multiple components, including electrodes, electrolytes, separators, and current collectors. The separator serves a similar purpose as it does in a battery by separating the two electrodes to avoid short-circuits and allowing ions to pass through. The fundamental concept behind energy storage in a supercapacitor involves storing electrical energy through the capacitance of the electric double layer, which results from charge separation at the interface between the electrolyte and bath solution.
